

Decreased injection frequency
Achieve sustained VA gains with as few as 3 injections in Year 2 1
EYLEA®: YOUR CHOICE FOR FOR PROACTIVE TREAT AND EXTEND UP TO Q161
Sustained VEGF supression with EYLEA®1

Mean duration of VEGF-A suppression with EYLEA®:67+/-14 days
Mean duration of VEGF-A suppression with ranibizumab 34+/-5days
Definite VEGF suppression
Uncertain suppression status
Uncertain end of suppression
VA GAINS MAINTAINED AT YEAR 2 WITH PROACTIVE EXTENDED DOSING2
Mean number of injections at 96 weeks for both groups: 10.4 injections2
ACHIEVE UNSURPASSED AND SUSTAINED VA GAINS WITH EYLEA®


- Proportion of patients achieving ≥12-week dosing at 96 weeks
- Proportion of patients achieving 16-week dosing at 96 weeks
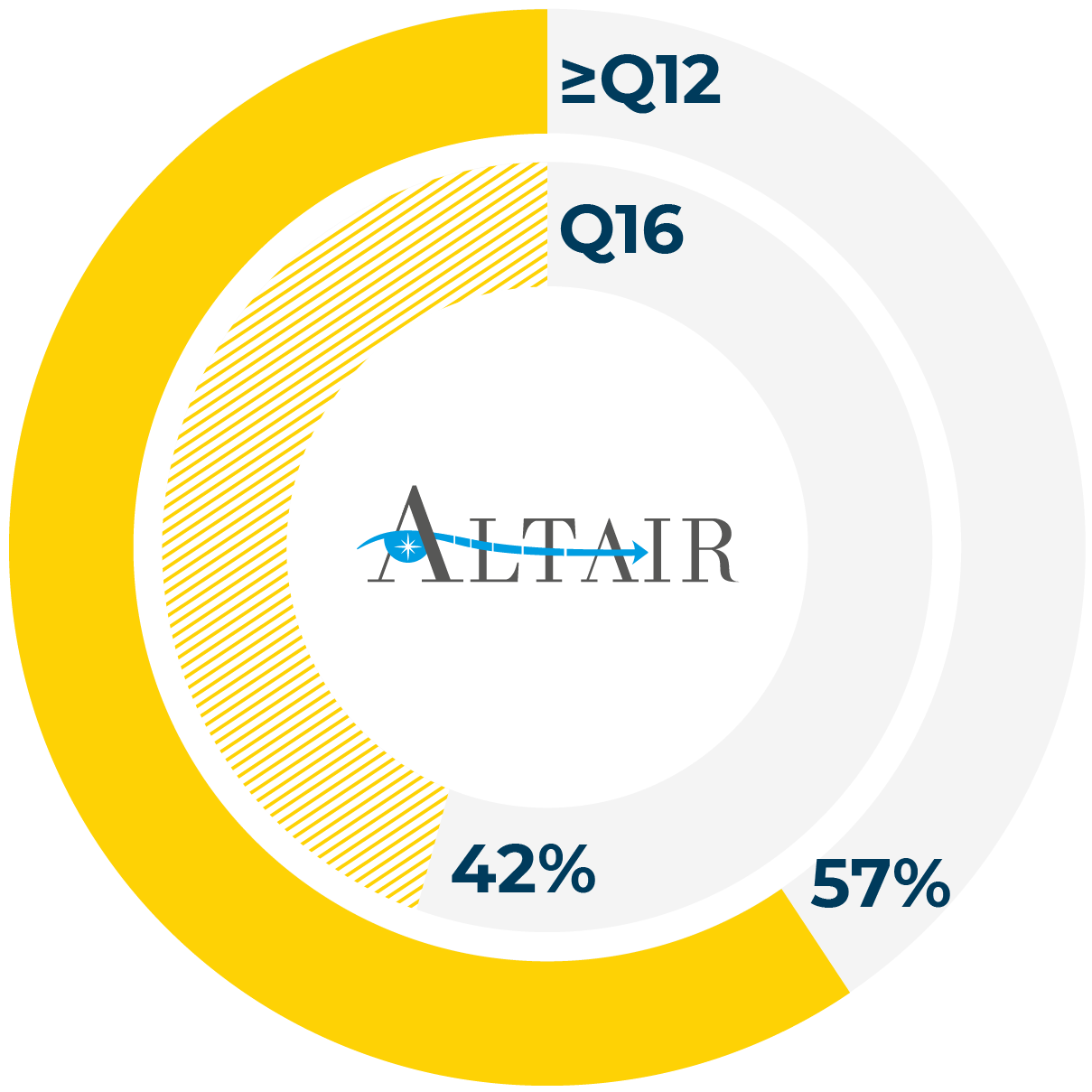

- Proportion of patients achieving ≥12-week dosing at 96 weeks
- Proportion of patients achieving 16-week dosing at 96 weeks
References:
- EYLEA® (aflibercept solution for injection) Summary of Product Characteristics. Berlin, Germany: Bayer Pharma AG. Return to content
- Ohji M, Takahashi K, Okada AA, Kobayashi M, Matsuda Y, Terano Y; for ALTAIR Investigators. Efficacy and safety of intravitreal aflibercept treat-and-extend regimens in exudative age-related macular degeneration: 52- and 96-week findings from ALTAIR: a randomized controlled trial [published online ahead of print February 3, 2020]. Adv Ther. doi: 10.1007/s12325-020-01236-x. Return to content
- Muether PS, Hermann MM, Dröge K, Kirchhof B, Fauser S. Long-term stability of vascular endothelial growth factor suppression time under ranibizumab treatment in age-related macular degeneration. Am J Ophthalmol. 2013;156:989-993. Return to content
- Fauser S, Muether PS. Clinical correlation to differences in ranibizumab and aflibercept vascular endothelial growth factor suppression times. Br J Ophthalmol. 2016;100:1494-1498. Return to content
- Fauser S, Schwabecker V, Muether PS. Suppression of intraocular vascular endothelial growth factor during aflibercept treatment of age-related macular degeneration. Am J Ophthalmol. 2014;158:532-536. Return to content
- Richard G, Monés J, Wolf S, et al. Scheduled versus pro re nata dosing in the VIEW trials. Ophthalmology. 2015;122:2497-2503. Return to content
- Papadopoulos N, Martin J, Ruan Q, et al. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap,ranibizumab and bevacizumab. Angiogenesis. 2012;15;171-185. Return to content









